in vitro diagnostic medical devices
 There is a wide range of ‘devices’ that are intended to be used in medical applications and the majority of these ‘medical devices’ will come under the Medical Devices Directive, but there are also two additional Directives that cover two specific areas; ‘In-Vitro’ and ‘Active-Implantable.’
There is a wide range of ‘devices’ that are intended to be used in medical applications and the majority of these ‘medical devices’ will come under the Medical Devices Directive, but there are also two additional Directives that cover two specific areas; ‘In-Vitro’ and ‘Active-Implantable.’
In Vitro is Latin for in glass, which gives a good clue to as to what is in scope; effectively the In Vitro Diagnostic Medical Devices (IVDMD) Directive applies to any medical device (as defined under the Medical Devices Directive) which is intended to be used for the examination of specimens, including blood and tissue donations, derived from the human body, solely or principally for the purpose of providing information:
– concerning a physiological or pathological state, or
– concerning a congenital abnormality, or
– to determine the safety and compatibility with potential recipients, or
– to monitor therapeutic measures
The Directive (98/79/EC, as amended), which came into force on the 7th June 2000 applies to both devices and accessories, which could be a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, equipment, or system, whether used alone or in combination of other devices.
Conformity Requirements
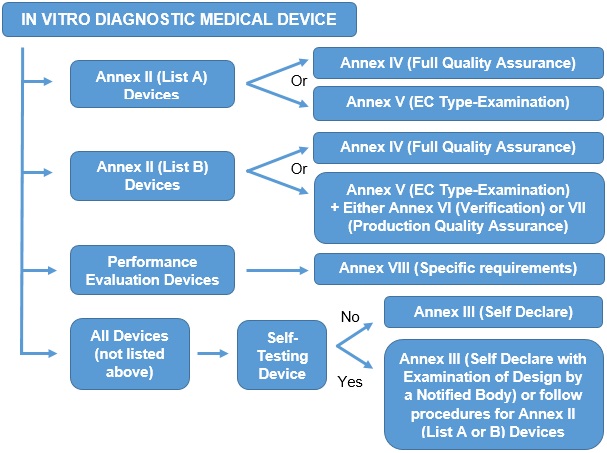
The procedure for CE marking is dependent upon the type of device and may need the involvement of a Notified Body; the types and procedures are summarised below:
Further Assistance
If you need help with CE marking In Vitro Diagnostic Medical Devices, then please contact the technical team on 01564 792349










